Article Contributed by Brittni Peterson, PhD and Emily Wozniak, PhD
Large-scale therapeutic products (many kg’s per year) command respect from contract manufacturers. These products generally consume dedicated manufacturing suites for multiple years and guarantee premium budgets. Their owners are often large pharmaceutical companies with significant resources and a track record of paying for the best.
Most Contract Development and Manufacturing Organizations (CDMOs) cater to these products because they are manufactured at a high price tag and come with guaranteed capacity utilization.
Small-scale therapeutic products generate less attention than their larger counterparts. These products typically face 18+ month waits for GMP suite availability with similar high costs as large-scale therapeutic products. Their owners are often small pharmaceutical companies with less resources and no track record within the industry.
Small-scale therapeutics are often given low priority by CDMOs and are generally considered unattractive, especially in a capacity-constrained market.
There is a growing need in the bioprocessing industry for CDMOs who cater to small biopharmaceutical companies and small-scale therapeutics manufacturing.
Who needs small-scale?
In the last decade, the biopharmaceutical industry has seen a shift towards biologics necessitating smaller batch sizes, including:
“Small, flexible CDMOs are able to fill a gap in the market.
- Preclinical and clinical trial material. With over 20,000 biologics in development worldwide, the global biopharmaceutical market is $200 billion, growing at a 15% CAGR. Fifty percent of biologics are in preclinical and another 30% are in clinical studies (BioPlan 2016), which require production in milligram to gram scales. This means 80% of biologics in development do not require large (kilogram) batches.
- Precision medicine and orphan diseases therapies. Because these therapies treat individual patients (precision medicine) or very small patient populations (orphan diseases; fewer than 6.37 people in 10,000), they may not require large batches, even at commercial scale. As a $130 billion market growing at 11% CAGR (Global Market Insights; Orphan Drug Report 2015), these therapies will continue to drive the need for small-scale biologic production.
- Recombinant proteins. Recombinant proteins, including chimeric IgGs, fusion proteins, bi- and tri- specific antibodies, antibody drug conjugates, currently comprise 65% of all biopharmaceuticals and make up a $130 billion market growing at a 12% CAGR (BioPlan 2015). Because of their complexity, these proteins are often “difficult-to-express” by the host cell line and challenging to produce at large-scale (for more see our previous post). These heavily engineered proteins also have improved targeting, which can decrease the necessary patient doses required. By necessity, these biologics are produced at smaller scales.
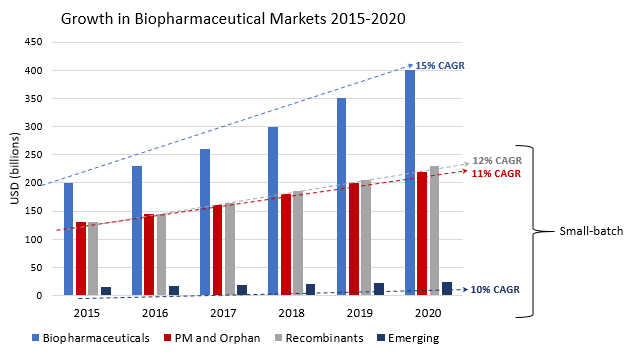
- Biosimilars or generics. As biopharmaceuticals come off patents, follow-on biologics like biosimilars (the “generic” form of a biologic) and biobetters (an improved version) are beginning to crowd the marketplace, creating worldwide competition over target patient populations. Currently there are 800 biosimilars and about 500 biobetters in development for a total of ~1,400 products aiming to replace just over 100 currently marketed molecules (BioPlan 2016). Growth in the pipeline is expected to continue and the competition of multiple companies producing the same drug will likely drive smaller batch sizes.
- Emerging markets. Many new markets throughout the world are emerging as centers for biopharmaceutical manufacturing. Many regions in South America, Africa, and the Middle East seek to produce biosimilars and other biologics regionally, cutting down their potential patient populations and thus the necessary production scales.
For more on small-scale manufacturing applications see previous articles in Cell Culture Dish here and here.
The Problem: Small-scale Biologics Manufacturing Is Cost-prohibitive
So, if biologics necessitating small production batches are so hot right now, then why aren’t big CDMOs interested in taking on these smaller projects?
Historically, GMP facilities were not designed with small-batch production in mind. Human IgGs have dominated the therapeutic market as an efficient, easily expressed product. Biopharmaceutical manufacturing platforms were designed and optimized to utilize large (2,000-5,000L) fed-batch bioreactors to cost-effectively produce high protein yields.
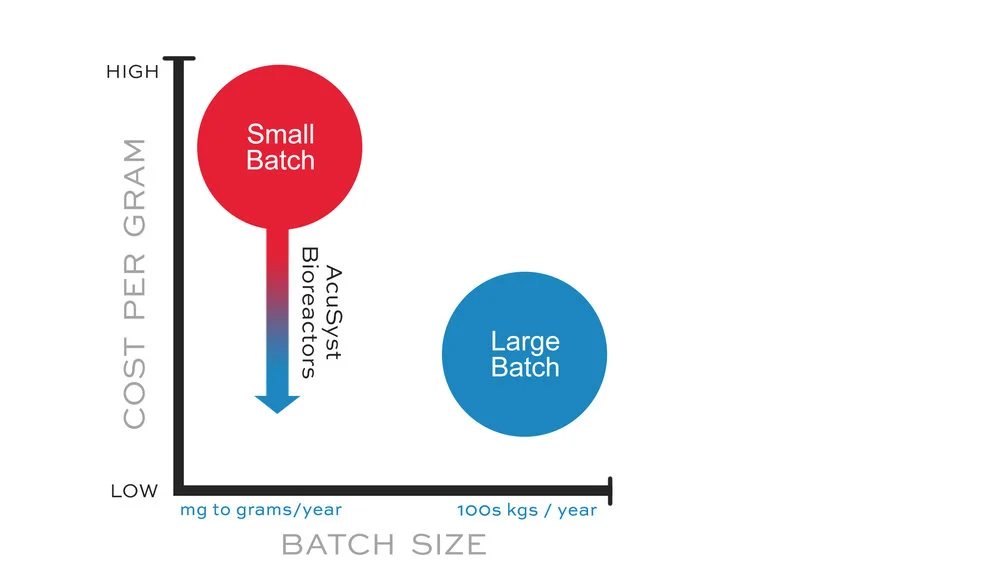
Large-scale platforms do not fit small-scale production needs and GMP facilities were not designed to maximize throughput of small batches. These inflexible large-scale platforms are unable to manufacture multiple products simultaneously (Walker N, 2016; Jacquemart et al 2016) and generate similar costs as the large batches (overhead, labor, facility, materials), even when a smaller batch size is required. Small-batch production using large-scale platforms can be very expensive per gram of product.
As the biologics industry rapidly grows, there is a high demand for additional biomanufacturing capacity (BioPlan 2016). And with GMP facilities in such high demand, CMOs can charge high prices and be selective about which projects they undertake.
New classes of therapeutics and new applications that require small-scale manufacturing are driving the need for flexible, cost-efficient biomanufacturing platforms. Fortunately, the industry is developing solutions.
The Solution: Fully Closed, Fully Automated, Small-footprint Systems
In the U.S., biologics account for roughly 40% of prescription drug spending, despite making up only 2% of all approved drugs (Jacquemart et al 2016). This is largely due to cost of goods (CoGs) related to manufacturing, which, as discussed above, is especially true for small-scale production.
One effective solution is to reduce the high overhead, labor, facility and raw material costs, which can be achieved through fully closed, fully-automated, small footprint manufacturing systems. These systems greatly reduce CoGs by roughly 30% (Jacquemart et al 2016). The smaller size of these bioreactors reduces the amount of facility space needed and raw materials required, which in turn decrease costs.
Automation further reduces labor requirements, decreasing costs and ensuring product quality and consistency from batch to batch. And, fully closed systems can be maintained in lower-classified GMP facilities that are not only cheaper to build and maintain, but enable facility flexibility for multi-product production to increase throughput. This results in faster production and decreased development time, which can help drive down the long waitlists at CDMOs.
In comparison to traditional stainless-steel technology, single-use technologies and flexible facility designs can reduce capital costs by 40-67% and operating costs by 20-30% (Walker N, 2016; Jacquemart et al 2016). See below for an example of how fully closed, fully automated, small footprint systems can decrease production costs compared with large stainless steel and single use stirred tank bioreactors.
“The industry is experiencing a capacity crunch, and there is up to a 12-18 month wait for GMP production.
Cost-efficient Manufacturing of Small-scale Biologics
Three Types of CDMOs
1. Too Hot: Large and Experienced
Small companies with small projects are a low priority for most large CDMOs that are already experiencing tremendous capacity constraints. Getting lost in the shuffle is a huge risk for a small company. So, don’t allow yourself to be overlooked by big CDMOs that are too busy to make your project a high priority.
2. Too Cold: Small and Inexperienced
If the product is complex or difficult to make, you need a small CDMO that can pay attention to your needs and provide technical expertise. Beware that some small CDMOs may not have enough experience to provide the technical expertise nor quality systems that you need. Make sure to thoroughly complete your due diligence.
3. Just Right: Small and Experienced
Consider Cell Culture Company for your small-batch bioproduction needs. Cell Culture Company has 25+ years of experience in process design and GMP manufacturing (20+ clinical trials supported). Our technology, expertise and size allow us to be more flexible than larger CDMOs. We take a hands-on approach with our clients and emphasize great customer service so our clients are involved throughout the entire manufacturing process. And…we have significantly lower wait times for GMP suites.
Cell Culture Company’s AcuSyst® bioreactors are fully closed, fully automated and small-footprint systems enable cost-effective small-scale manufacturing.
“Technology, custom manufacturing experience, and right-sized operations are the ingredients that enable us to provide high quality, small scale GMP biologics manufacturing at a competitive price.
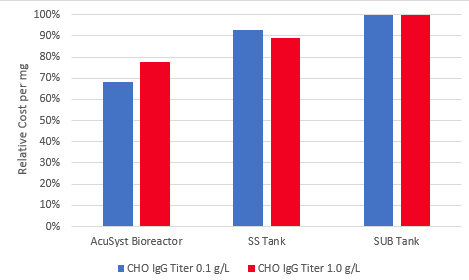
We calculated the costs of producing IgG from a high-expressing CHO line (1.0 g/L) and a low-expressing CHO line (0.1 g/L) for AcuSyst perfusion compared to stainless steel and single-use stirred tanks (See our article in BioProcess International for how we calculated these costs). We found that AcuSyst bioreactors provided a 15-30% reduction in costs compared with these large-scale bioreactors. Cost savings with AcuSyst bioreactors are accomplished by a reduction in facility requirements, disposable costs, labor, raw materials, and process steps. To learn more about how AcuSyst bioreactors decrease costs, see our recent article in Genetic Engineering and Biotechnology News.
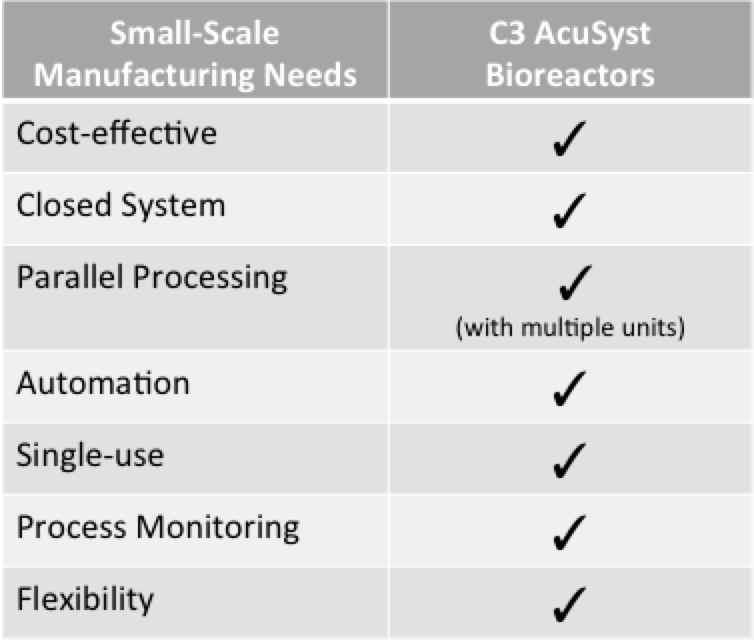
In addition to cost-effective manufacturing, AcuSyst bioreactors offer benefits of automated process monitoring and continuous perfusion of nutrients and waste removal. These features extend cell line health and production lengths and ensure that products are high quality and consistent from batch to batch. To learn more, see our recent publication in BioProcess International.
Cell Culture Company is not too hot, or too cold, we’re just right for your small batch manufacturing needs. Contact us here to start a conversation about using AcuSyst bioreactors and Cell Culture Company’s CDMO services.
