Get more out of your cell line
Previous studies have suggested that perfusion culture may be able to overcome some of the limitations of difficult-to-express proteins. To test this directly, we grew hybridoma cells in AcuSyst perfusion bioreactors expressing protein products that were known to be challenging to produce in shaker flask culture.
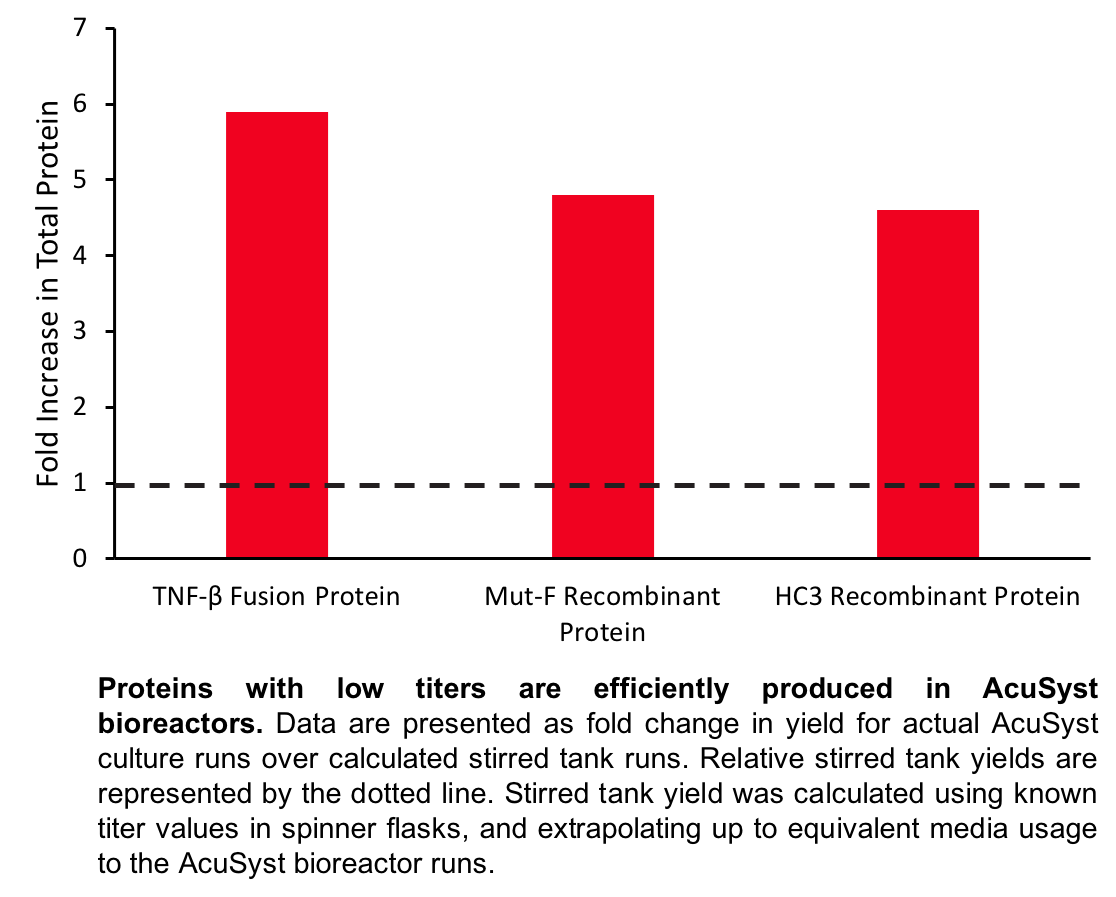
Case Study 1: TNF-beta fusion protein
A TNF-β fusion protein was known to have a low titer of 0.023 mg/L when grown in shaker flask culture. In an attempt to increase protein titer and to extend the culture run length to maximize productivity, TNF-β protein was produced in a two-cartridge AcuSyst bioreactor (Maximizer) over a 70 day culture run. A total of 400 L of media was consumed. In the EC space, 5 liters of supernatant was collected containing 54 total mg of protein.
Case Study 2: Mut-F recombinant protein
An anti-HIV-1 replication recombinant protein (Mut-F) was expressed by a CHO cell line in shaker flask culture at a titer that was too low for efficient manufacturing (0.012 mg/L). The cell line was grown in a six-cartridge AcuSyst bioreactor (Xcellerator) for 77 days to maximize productivity. A total of 3,850 L of media was consumed throughout the run, and 15 L of EC space supernatant was collected containing 220 total mg of Mut-F protein.
Case Study 3: HC3 recombinant protein
The recombinant protein HC3, expressed by CHO cells, was observed to cause suppression of expression at titers greater than 10 mg/L in shaker flask. We proposed that higher titers might be achieved in a perfusion culture in which protein is continuously harvested. The cell line was grown in a one-cartridge AcuSyst bioreactor (AutovaxID) for 30 days, and consumed a total of 100 L of media. In the EC space, 10 liters of supernatant was harvested containing 4.6 total grams of HC3 protein.
